Post Views:
4,243
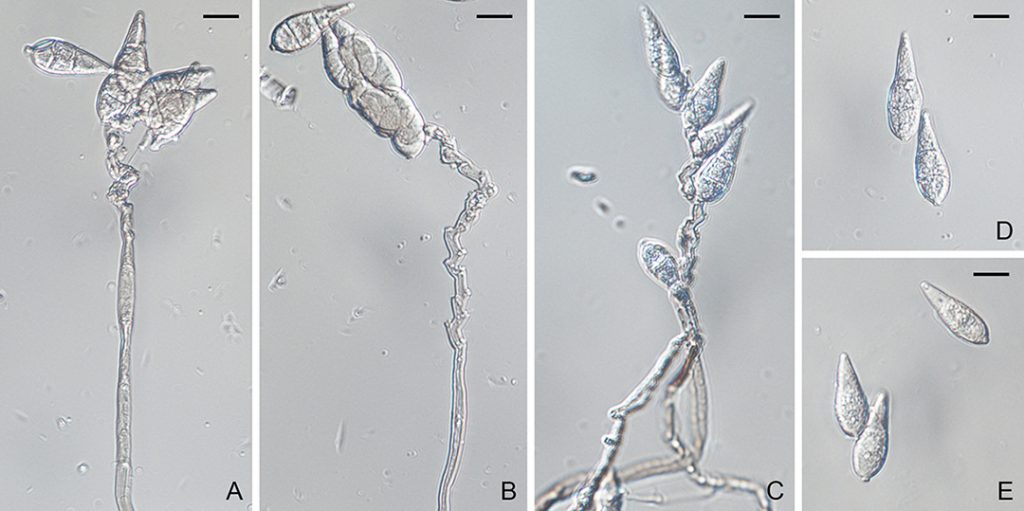
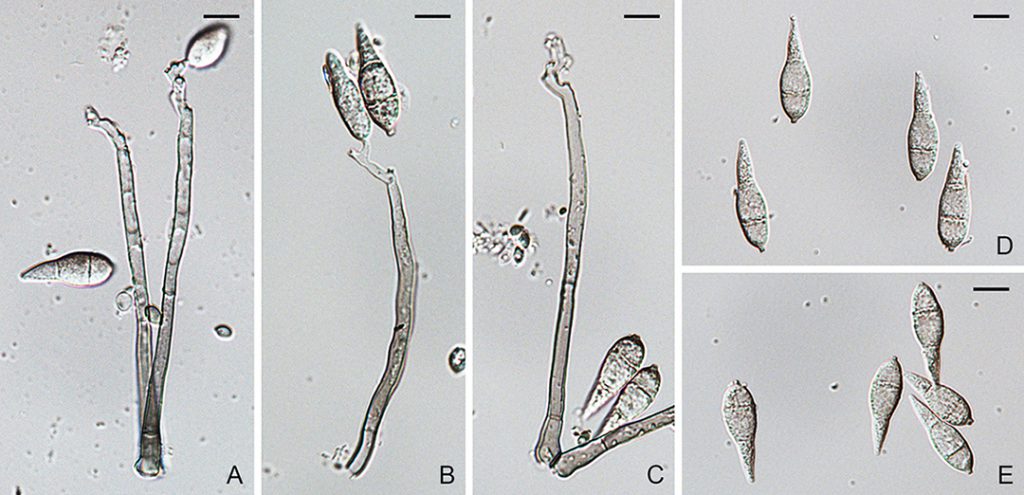
Figure. Pyricularia grisea (BPI 881984). A–E. Conidiophores and conidia. Scale bars: A–E = 10µm.
Pyricularia grisea Cooke ex Sacc., Michelia 2(no. 6): 20 (1880).
MycoBank: MB224559.
≡ Trichothecium griseum (Cooke ex Sacc.) Cooke, in Ravenel, Amer. Fungi: no. 580 (1881).
≡ Dactylaria grisea (Cooke ex Sacc.) Shirai, in Miyake, J. Coll. Agric. imp. Univ. Tokyo 2: 262 (1910).
= Magnaporthe grisea (T.T. Hebert) M.E. Barr, Mycologia 69:954 (1977).
≡ Ceratosphaeria grisea T.T. Hebert, Phytopathology 61:86 (1971).
≡ Phragmoporthe grisea (T.T. Hebert) M. Monod, Beih. Sydowia 9: 153 (1983).
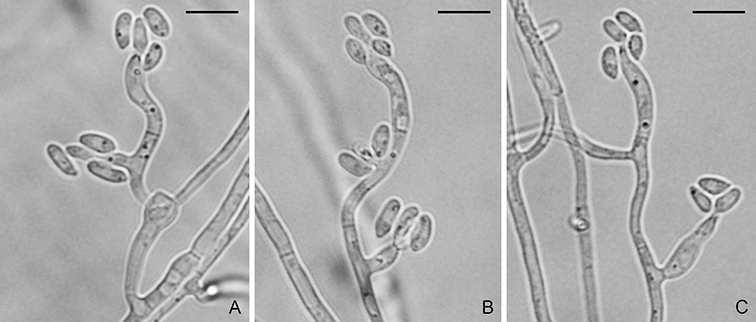
Ascomata perithecial, superficial or immersed, solitary or gregarious, dark brown to black, 60–300 µm diam, with a cylindrical, hyaline to light brown neck, 60–150 × 100–1200 µm. Paraphyses septate, hyaline, dissolving at maturity. Asci 8-spored, unitunicate, clavate, 7–10 × 55–90 µm, with a refractive ring. Ascospores 1–3-seriate, fusiform, straight or slightly curved, 3-septate, not or slightly constricted at septum, hyaline, smooth, 4–7 × 17–24 µm (Sexual state description from Hebert, 1971). Conidiophores solitary, erect, straight or curved, unbranched, 1–3-septate, brown, smooth, 100–135 × 3–6.5 µm. Conidiogenous cells integrated, terminal or intercalary, light brown, smooth, 80–110 × 3–6 µm, with several protruding denticles, 0.5–1.5 × 1–2 µm. Conidia solitary, pyriform to obclavate, 2-septate, light brown, smooth to finely roughened, 24–35 × 7–11 µm, with a truncate hilum, 0.5–1.5 × 1–2 µm.
Typification: Lectotype of Pyricularia grisea BPI, Ellis & Everhart, North American Fungi no. 374 (Rossman et al., 1990). Epitype of P. grisea CBSH-22280 (Crous et al., 2015a). Ex-epitype culture of P. grisea CBS138707 (US0043) (Crous et al., 2015a). Holotype of “Magnaporthe grisea” BPI625033 (Hebert, 1971).
Gene sequences: JX134656 (18S), JX134670 (ITS), JX134682 (28S), KM485187 (ACT), KM485258 (CAL), JX134710 (MCM7), JX134724 (RPB1), JX134696 (TEF1).
Genome sequences: PRJEB7653 (genome), SRX798638 (transcriptome).
Specimens examined: USA, Georgia, on Digitaria sanguinalis, Aug. 2000, L.P. Tredway, 1120-20 (BPI881983), 1100-4 (BPI881984); Indiana, on Digitaria, 3 Jun. 2011, J. Xu, M63; New Jersey, on Digitaria, 2 Aug. 2013, J. Luo and N. Zhang, RUTPPpg130802a, RUTPPpg130802d.
Hosts/substrates: On Digitaria, Echinochlos crus-galli var. frumentacea, and Lollium perenne (Poaceae).
Distribution: Worldwide.
Notes: Saccardo (1880a, b) first proposed Pyricularia grisea for a species from Digitaria sanguinalis in New Jersey. Cavara (1892) subsequently introduced P. oryzae for another species from Oryza sativa in Italy. These two species are morphological similar. Historically P. grisea was applied to grass and other host isolates, while P. oryzae to rice isolates. According to morphology and success interfertility among isolates from various hosts, Rossman et al. (1990) synonymized P. oryzae under P. grisea. However, Couch and Kohn (2002) revealed generic distinction between them based on multigene phylogeny and RFLP assay. They proposed P. grisea for the crabgrass isolates, and P. oryzae for rice and other grass isolates. Klaubauf et al. (2014) further investigated the phylogeny of Pyricularia and refined definitions of both species. Currently P. grisea includes isolates mostly on Digitaria, as well as few on Echinochlos crus-galli var. frumentacea and Lollium perenne. Pyricularia grisea can cause leaf spot or leaf blight diseases on Digitaria.
Copyright 2022 by The American Phytopathological Society. Reproduced, by permission, from Luo, J., and Zhang, N. 2022. The Rice Blast Fungus and Allied Species: A Monograph of the Fungal Order Magnaporthales (https://my.apsnet.org/APSStore/Product-Detail.aspx?WebsiteKey=2661527A-8D44-496C-A730-8CFEB6239BE7&iProductCode=46826). American Phytopathological Society, St. Paul, MN.